At Chiba University, a groundbreaking technology is being developed by Professor Yasuo Izumi’s group from the Graduate School of Science. This remarkable innovation aims to “recapture carbon dioxide (CO2) from the air and transform it into valuable substances beneficial to human beings.” By utilizing cost-effective photocatalysts* and natural sunlight, the process efficiently converts CO2 into fuels and raw materials for the chemical industry. Professor Izumi’s research has garnered significant attention as a promising approach toward achieving a new carbon-neutral cycle, driving the realization of a sustainable future.
*A photocatalyst is a substance that reacts in the presence of light. It serves as a catalyst, which facilitates a chemical reaction by lowering the energy required for the reaction and enhancing the reaction rate.
Pursuing Ideal Photocatalysts with a “Unique” Research Policy
Please tell us about your specialty and research focus.
My specialty lies in the field of surface chemistry. Specifically, I dedicate myself to exploring valuable catalytic reactions occurring on solid surfaces and investigating their reaction mechanisms utilizing state-of-the-art analytical methods.
In recent years, our research has been primarily focused on the capture of CO2 from the air and its conversion into fuels or resources, such as raw materials for plastics. This research aims to establish a novel carbon-neutral cycle.
Central to the advancement of this research is the crucial role of a substance known as a “Photocatalyst.”
What is “Photocatalyst?”
Photocatalysts are substances that induce specific chemical reactions when exposed to light. When light interacts with a photocatalyst, the “electrons” within the catalyst absorb energy and become active, while positively charged spaces, known as “holes,” are simultaneously created in the locations where the electrons originally resided. These “electrons” and “holes” play a crucial role in driving photocatalytic reactions. Various chemical reactions take place when “electrons” move towards surrounding substances (reduction) or when electrons from surrounding substances migrate into the holes (oxidation).
This photocatalytic reaction can effectively break down harmful substances in the air, odor-causing compounds, and microorganisms, such as bacteria and viruses. It finds applications in various areas, including air purifiers, vending machines, public restrooms, building exterior walls, or roofing materials. Additionally, it has the potential to decompose air pollutants, thereby contributing to the improvement of urban environments.
In my research, I employ photocatalysts to reduce CO2 and convert it into other substances.
I see. Can you tell me more about your photocatalysts research?
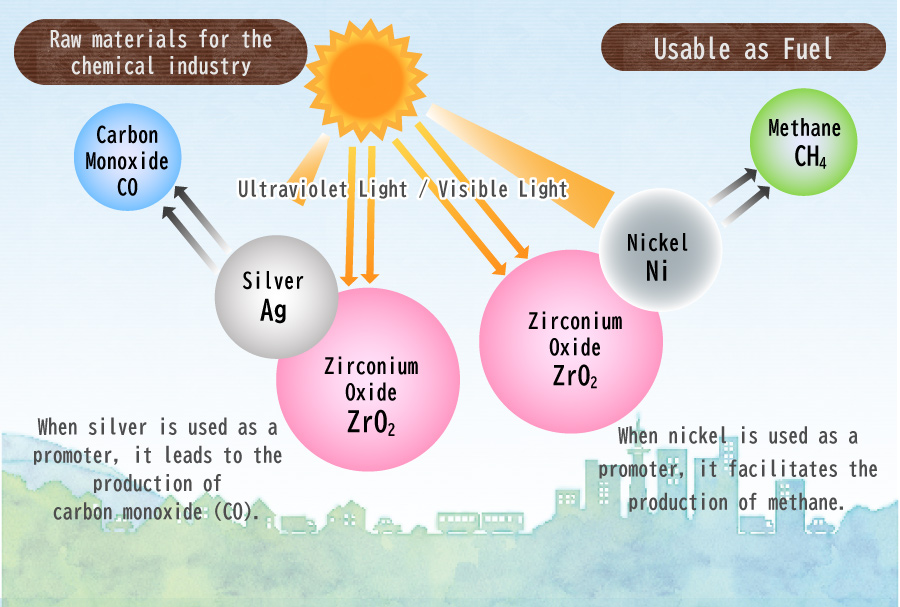
I am currently conducting research involving the utilization of a photocatalyst called Zirconium Oxide (ZrO2). When exposed to ultraviolet light with a wavelength of 248 nm or less, ZrO2 undergoes a reaction that effectively reduces CO2.
However, the effectiveness of ZrO2 alone in this reaction is limited. To enhance the reaction’s efficiency, a substance called a “promoter” is required. Further investigations have revealed that the choice of promoter significantly affects the resulting product. For instance, when silver (Ag) is employed as a promoter, carbon monoxide (CO) is produced, which is widely used as a feedstock in the chemical industry. On the other hand, the use of nickel (Ni) as a promotor leads to the generation of methane (CH4), a valuable fuel source.
In addition, I have recently been focusing on the production of relatively complex compounds, including ethylene (C2H4), ethane (C2H6), propylene (C3H6), and propane (C3H8), utilizing cobalt as a promoter. Ethylene and propylene are highly versatile substances that can serve not only as fuels but also as necessary raw materials for the production of plastics.
You indeed contribute to the production of various useful substances!
In addition to the production of valuable substances, my research goes beyond employing theoretical calculations and spectroscopy to gain a detailed understanding of each step of the photocatalytic reaction process.
While many researchers focus primarily on the production of useful substances, my approach can be described as “Unique.” By unraveling the intricacies of the catalytic reaction process, I can contribute to the design of new catalysts with enhanced activity. I feel that this research methodology is crucial for advancing catalyst development, a pivotal aspect of building a sustainable society.
From Petroleum to Carbon Neutral
Have you been engaged in carbon-neutral research for an extended period?
No. Initially, I focused on catalysts that produce useful substances from petroleum and ones that decompose nitrogen oxides. However, as environmental sustainability initiatives gained momentum, I made a conscious decision to shift my research toward renewable energy.
Among the various forms of renewable energy, I opted for light as it offered better control over the progress of catalytic reactions. Light, composed of energy particles called “photons,” allows for a step-by-step advancement of catalytic reactions each time a photon interacts with a photocatalyst. This property allows for easier control of catalytic reactions, enabling them to start and stop, and facilitating analysis of the reaction process, providing more flexibility in controlling the reaction rate.
While heat could be an alternative to light, it is not sustainable due to the requirement of high temperatures and the associated energy needed to maintain them. Photocatalysis presents an appealing avenue of study that not only facilitates research but also holds great potential for realizing a sustainable society.
Towards advancing the development of a more efficient photocatalyst

What stage is your research currently at?
As mentioned earlier, I am currently focusing on the development of a photocatalyst for the production of methane, ethylene, and propylene from CO2. Previously, I could achieve a reaction rate of 0.1 millimol per hour per gram of catalyst. However, through my ongoing research, I have realized significantly improved efficiency, now obtaining products at a rate of approximately 1 millimol per hour per gram of catalyst (maximum value), which is ten times faster than before.
What are your future research goals?
Our primary goal is to develop a photocatalyst with even higher performance compared to the current one. To accomplish this, I need to advance my research in catalyst structure construction and reaction control. For instance, we aim to create a semiconductor (photocatalyst) with only a few atomic layers and deliberately engineer reaction sites* that facilitate the efficient reduction of CO2. Additionally, I would like to explore alternative catalysts to zirconium oxide that exhibit high efficiency.
*A specific part of catalyst components that actually participates in the reaction. It is also referred to as an “active site” or “active point.
In the coming years, I plan to establish a computer system capable of identifying the optimal photocatalyst based on the extensive experimental data we have accumulated.
Pursuing research while seeking compatibility with conventional industries
What challenges do you face in implementing your research findings into practical applications?
I firmly believe that the practical applications of my research rely on the development of a high-performance and cost-effective photocatalytic system. The key objective is to enhance the conversion efficiency of CO2 into valuable substances such as methane, ethylene, ethane, propylene, propane, methanol, or ethanol. Thus, it is crucial for me to maintain a thorough focus on advancing my research efforts.
Once my research findings are successfully translated into practical applications, they hold the potential to facilitate the direct conversion of CO2 emitted by steel mills and chemical plants into useful substances on-site. I consider the integration of such a method into existing facilities as the initial step toward practical implementation.
Lastly, what do you believe is crucial for the establishment of a sustainable society?
In the current landscape, numerous companies are diligently working towards transitioning to renewable projects. However, cost considerations often pose a significant challenge in this endeavor. Achieving the right balance between environmental protection and cost-effectiveness is a major hurdle in the pursuit of a renewable energy society.
Given these circumstances, it becomes essential to envision a novel paradigm for the renewable energy industry, one that acknowledges its distinctiveness from conventional sectors. Naturally, my research is aligned with this objective.
While there may be numerous difficulties to overcome, I remain unwavering in my commitment to advancing my research, guided by the principle of “Advancing a Sustainable Society with Chemistry.”
Series
The Role of Universities to Achieve Carbon Neutrality
What role should universities play in realizing carbon neutrality by 2050? This series will explore this question, accompanied by research conducted at Chiba University.
-

#1
2023.06.22
Harnessing CO2 with a Photocatalyst for Fuel and Plastic Feedstocks: Advancing a Sustainable Society with Chemistry
-

#2
2023.06.29
Unlocking Untapped Urban Resources: Driving Carbon Neutrality through Life Cycle Assessment
-

#3
2023.07.04
Predicting Future CO2 Absorption and Emissions in Terrestrial Ecosystems: International Geostationary Meteorological Satellite Network for Accurate Estimation
-

#4
2023.07.12
The Global Goal of Carbon Neutrality by 2050 (Part 1): Universities as Agents of Change
-

#5
2023.07.14
The Global Goal of Carbon Neutrality by 2050 (Part 2): A decarbonized society from a local perspective
-

#6
2023.09.12
Reassessing the role of “Forest Resources” in achieving carbon neutrality: Measuring forests with the combination of drones, mathematics, and computer graphics
-

#7
2023.09.28
Navigating Offshore Wind Power Expansion: Nurturing Ocean-based Wind Energy Management Experts through Industry-Academia Partnership
Recommend
-

From ELSI to RRI: Exploring the Ethical Landscape of Science and Technology
2024.11.21
-

Designing a world-class climate-resistant fruit!~Developing “Designed Grapes” for Custom-Made Wine to Commemorate Special Occasions
2022.11.07
-
-200x150.jpg)
Research is a Role-Playing Game-Innovation in Drug Discovery Stems from the Ability to Think Deeply
2022.11.07