Researchers dive deep into the gelation mechanisms of widely used thermoresponsive materials, paving the way for smart medicines and cosmetics.
Thermoresponsive mixtures made from poloxamers are widely used in drug delivery, as they remain liquid at room temperature but solidify in response to body heat. However, their gelation behavior is difficult to control. Now, researchers have investigated how mixtures of poloxamers P407 and P188 behave at different temperatures and concentrations. Using a comprehensive approach, they uncovered the molecular mechanisms behind their gelation processes, providing insights into the development of smart therapeutic formulations.
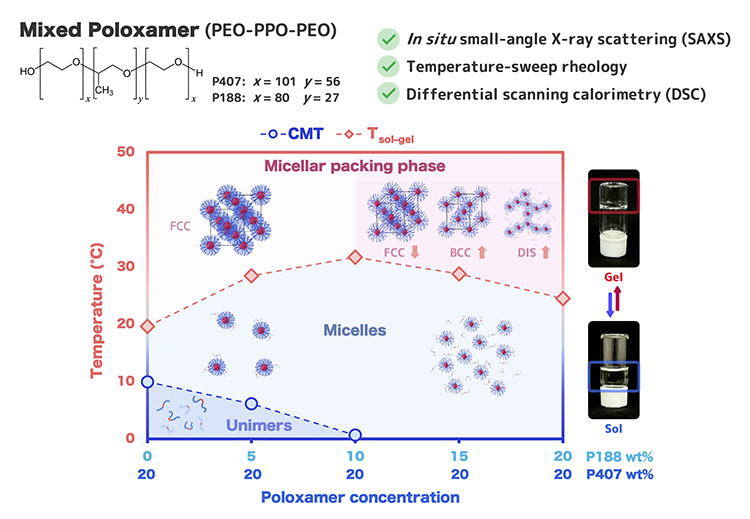
Image title: Study on how the behavior of poloxamer mixtures evolves depending on temperature and concentration
Image caption: Schematic illustration of the temperature-dependent self-assembly and sol–gel transition behavior of mixed poloxamer 407 (P407) and poloxamer 188 (P188) systems. The diagram summarizes the evolution from unimers to micelles and further micellar packing upon heating, highlighting the non-straightforward shifts of the sol–gel transition temperature (Tsol–gel) as a function of P188 content. The proposed mechanism is supported by in situ small-angle X-ray scattering (SAXS), temperature-sweep rheology, and differential scanning calorimetry (DSC), linking molecular self-assembly to macroscopic gel formation.
Image credit: Associate Professor Kenjirou Higashifrom Chiba University, Japan
Image license: Original content
Usage restrictions: Cannot be reused without permission
In the world of modern medicine, most people focus on the active pharmaceutical ingredients, which are the chemicals that specifically fight a disease’s symptoms or causes. However, the unsung heroes of pharmacy are excipients—substances formulated alongside the active ingredients to ensure they reach the right part of the body at the right time. Simply put, excipients are as vital as the drugs themselves because they provide a controllable means of administration. A prominent example is thermoresponsive compounds that enable in situ gelling. These smart liquids, once administered to the body, transform into a solid gel in response to body heat, enabling the medication to stay exactly where it is put and providing a localized, steady release.
Poloxamers are polymeric materials that are among the most trusted tools for achieving in situ gelling and are widely used in topical creams, eye drops, and even oncological drug applications. Despite their popularity, working with these materials can be challenging; even the most researched variety, poloxamer 407 (P407), is notoriously finicky. The exact temperature at which P407 turns from liquid to solid gel, called the sol–gel transition temperature, fluctuates depending on its concentration. This makes P407 difficult to ensure a formula that stays liquid in a cool syringe or vessel but solidifies reliably once it touches warm human body. Although P407 can be mixed with poloxamer 188 (P188) to finely tune its transition temperature, the underlying physics governing this behavior remain unclear.
Against this backdrop, a research team led by Associate Professor Kenjirou Higashi from the Graduate School of Pharmaceutical Sciences, Chiba University, Japan, conducted a deep dive into the structural evolution of P407/P188 mixtures. Their paper, made available online on November 17, 2025, and published in Volume 705 of the Journal of Colloid and Interface Science on March 01, 2026, explores the fine molecular and macroscopic changes that occur when these two polymers interact at different temperatures. This work was co-authored by Dr. Prakasit Panyamao (a PhD student in Chiba University and a lecturer in Chiang Mai University, Thailand), Dr. Chalermpong Saenjum from Chiang Mai University, and Dr. Keisuke Ueda and Dr. Kunikazu Moribe from Chiba University.
“Our research was motivated by repeated observations that small changes in poloxamer composition can cause unexpectedly large and inconsistent changes in gelation behavior. The lack of a clear mechanistic explanation prompted us to investigate how molecular self-assembly translates into macroscopic gelation, with the aim of moving beyond trial-and-error formulations,” explains Dr. Panyamao.
To achieve their goal, the team used a sophisticated three-pronged approach that revealed key properties at different scales. They used differential scanning calorimetry to measure heat changes at the molecular level, rheology to test the stiffness and sol–gel transition temperature of the gels, and in situ synchrotron small-angle X-ray scattering to determine their internal crystalline architecture as it formed.
Through detailed analysis, the team discovered that P188 at low concentrations essentially acts as a disruptor, preventing P407 molecules from packing together and thus raising the gelation temperature. However, at higher concentrations, P188 competes for water molecules with P407. This forces P407 to dehydrate faster and form a large number of tiny spherical structures called micelles. These micelles become so tightly packed that the mixture turns into a gel at lower temperatures. Taken together, the results provide the first comprehensive map of how these poloxamers reorganize from a disordered solution state into highly organized, crystal-like lattices with distinct arrangements.
These insights into the gelation processes of P407/P188 mixtures are crucial for the pharmaceutical industry, as they will enable scientists to better customize the thickness and stability of thermoresponsive gels to suit specific medical needs. “The findings in this study support the precise design of sustained-release formulations for localized therapies in ophthalmology, dermatology, and oncology, where controlled gelation can enhance drug retention and minimize side effects,” highlights Dr. Panyamao.
Beyond clinical applications, the implications of this work reach into other industries that require materials to change shape or texture in response to temperature. “This research could not only help create safer, more predictable, and more accessible gel-based medicines, but also bolster the development of smarter materials in cosmetics, food, and technology,” concludes Dr. Higashi.
To see more news from Chiba University, click here.
About Associate Professor Kenjirou Higashi from Chiba University
Dr. Kenjirou Higashi started his academic career as an Assistant Professor in 2007 and obtained a Ph.D. in Pharmaceutical Science from Chiba University in 2011. He currently serves as an Associate Professor at the Graduate School of Pharmaceutical Sciences. He specializes in molecular pharmaceutics, drug solubility and stability, pharmaceutical nanoparticles, and their physicochemical analysis. He has published over 160 papers on these topics. He is also affiliated with academic societies such as the Pharmaceutical Society of Japan and the Academy of Pharmaceutical Science and Technology, Japan.
Funding:
This work was partially supported by the JSPS KAKENHI Grant-in-Aid for Scientific Research (B) (grant number 24K02162) and the National Research Council of Thailand (grant number N41A650087).
Reference:
Title of original paper: In situ SAXS and rheological correlation of microstructural evolution during the sol–gel transition of Poloxamer 407 modulated by Poloxamer 188
Authors: Prakasit Panyamao1,2,3, Kenjirou Higashi1, Keisuke Ueda1, Chalermpong Saenjum2,3, and Kunikazu Moribe1
Affiliations:
- Graduate School of Pharmaceutical Sciences, Chiba University, Japan
- Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Thailand
- Research Center for Innovation in Analytical Science and Technology for Biodiversity-Based Economy and Society (I-ANALY-S-T_B.BES-CMU), Multidisciplinary Research Institute (MDRI), Chiang Mai University, Thailand
Journal: Journal of Colloid and Interface Science
DOI: 10.1016/j.jcis.2025.139496
Contact: Kenjirou Higashi
Graduate School of Pharmaceutical Sciences, Chiba University
Email: ken-h@faculty.chiba-u.jp
Academic Research & Innovation Management Organization (IMO), Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: cn-info@chiba-u.jp
International Relations Officer, Faculty of Pharmacy, Chiang Mai University
Address: 239 Suthep road, Suthep, Mueang, Chiang Mai, Thailand
Email: inter.pharcmu@gmail.com









