The intranasal vaccine shows promise as a non-invasive therapeutic option for women battling cervical cancer
Cervical cancer, one of the most common cancers in women, is often treated with surgery, radiation, or chemotherapy, which can affect fertility and quality of life. Researchers at Chiba University have developed a new nasal therapeutic vaccine as a non-invasive treatment option. In animal studies, the vaccine produced strong and lasting immune responses against cervical tumors. If proven effective in humans, it could provide women with a safer, fertility-preserving alternative to current cancer treatments.
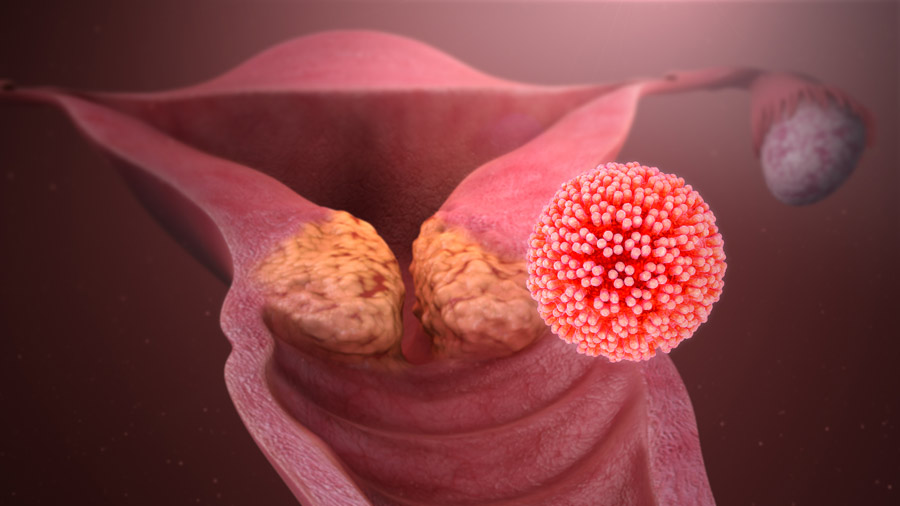
Image title: Nasal vaccine shows promise as a non-invasive treatment for cervical cancer
Image caption: The experimental nasal vaccine developed at Chiba University stimulated strong and long-lasting immune responses in animal models, activating tumor-fighting cells in the cervix and slowing cervical tumor growth. This approach offers a promising new direction for cervical cancer treatment, moving beyond conventional therapies.
Image credit: “HPV causing cervical cancer” by www.scientificanimations.com
Source link: https://openverse.org/image/b8b3c820-8a63-4814-a902-17d5a2da8730?q=cervical+cancer&p=2
Image license: CC BY-SA 4.0.
Usage restrictions: Credit must be given to the creator. Adaptations must be shared under the same terms.
Cervical cancer, which affects the reproductive tract, is one of the most common cancers in women worldwide. It is primarily caused by the human papillomavirus (HPV), a viral infection that spreads through sexual contact. While regular screening tests and preventive HPV vaccines can lower the risk of developing the disease, there are no approved therapeutic medicines to treat existing infections or HPV-associated cancers. As a result, current treatments remain limited to surgery, radiotherapy, or chemotherapy.
Now, researchers from Chiba University, Japan, have developed a therapeutic HPV vaccine that can be administered through the nose. This new intranasal vaccine could provide a non-invasive treatment option for individuals already infected with the virus. Unlike injectable vaccines, nasal vaccines trigger an immune response at the mucosal surface, which lines the upper airway and contributes to the protection of distant mucosal sites, including the reproductive tract, and acts as a protective barrier against pathogens and foreign particles.
The study, led by Associate Professor Rika Nakahashi-Ouchida and Ms. Hiromi Mori from Chiba University Hospital, Chiba University, was published in the journal Science Translational Medicine on November 12, 2025.
The team had previously found that nasal immunization produced strong immune responses in the reproductive tract against herpes simplex virus type 2 (HSV-2). Antigen-specific T cells remained in the vaginal mucosa, providing local protection where it was needed the most. Building on this idea, the researchers used cationic nano-sized hydrogel particles called cCHP nanogels to deliver HPV antigens directly to nasal mucosal tissues. These nanogels are made of positively charged cholesteryl groups that form small spherical structures. Their positive charge allows them to adhere to negatively charged nasal mucosal surfaces, where they gradually release the antigens.
“We have developed an intranasal therapeutic vaccine as a non-surgical alternative to conventional treatments that can compromise women’s quality of life. This novel nasal vaccine activates the mucosal homing pathways of lymphocytes, allowing it to trigger an immune response in the cervical mucosa, a site from the nasal administration,” says Associate Prof. Nakahashi-Ouchida.
The vaccine targets E7 oncoprotein, which is produced by HPV16, one of the most common high-risk strains associated with cervical cancer. This protein inactivates the key tumor suppressor pRb, which normally helps prevent cancer formation. To strengthen the immune response, the researchers added cyclic-di-AMP (c-di-AMP), an immune-boosting adjuvant that enhances T-cell-mediated immunity, encouraging the body’s T cells to attack infected or cancerous cells directly.
The resulting formulation, called cCHP-E7 + c-di-AMP, showed strong antitumor activity in both mice and macaques. In mouse models, the nasal vaccine significantly slowed tumor growth compared with control groups. The team then tested the vaccine in macaques using a nasal spray device that can also be used in humans. After four doses, the vaccinated macaques developed high levels of E7-specific helper and killer T cells that produced immune molecules associated with tumor control. The control animals did not show this immune response. Importantly, immune activity was also detected in the cervical tissue, suggesting that the nasal vaccine successfully triggered protection where it is most needed. Even 4 months after the final dose, E7-specific killer T cells remained active, indicating a lasting immune defense against HPV-related cancer.
According to the World Health Organization, cervical cancer caused an estimated 660,000 new cases and 350,000 deaths in 2022. If proven safe and effective in humans, this nasal vaccine could transform cervical cancer treatment by offering a non-invasive, fertility-preserving alternative to surgery. Moreover, the cCHP nanogel delivery system could serve as a platform for nasal vaccines targeting other pathogens as well as broader clinical applications.
“Immunotherapies such as intranasal therapeutic vaccines may help establish a new category of non-invasive treatment. These approaches could be extended to recurrence prevention and chronic disease management, offering patients safer and more accessible options,” says Associate Prof. Nakahashi-Ouchida.
To see more news from Chiba University, click here.
About Associate Professor Rika Nakahashi-Ouchida from China University, Japan
Rika Nakahashi-Ouchida is an Associate Professor in the Department of Human Mucosal Vaccinology at Chiba University, Japan. Her research focuses on understanding mucosal immunity and developing oral and nasal vaccines for infectious and immune-related diseases. She has led pioneering work on next-generation mucosal vaccines. Her recent studies include rice-based oral cholera vaccines and nanogel-based nasal vaccine delivery systems in mice and non-human primates.
Funding:
This research was supported by HanaVax Inc., the Japan Agency for Medical Research and Development under Grant No. 20im0210623 (to Hiroshi Kiyono), JP24ae0121040 (to Hiroshi Kiyono), JP223fa627003 (to Hiroshi Kiyono), the GAP Fund program of the University of Tokyo (to Rika Nakahashi-Ouchida), Japan Society for the Promotion of Science under Grant No. 20K20495 (to Kohtaro Fujihashi), 20H03856 (to Kohtaro Fujihashi), Chiba University-UC San Diego Center for Mucosal Immunology, Allergy and Vaccines (cMAV) Program (to Hiroshi Kiyono), Chiba University and Shionogi Human Mucosal Vaccinology Program (to Hiroshi Kiyono), and NIH NIDDK grants P30 DK120515 and R01 DK051677 (to Hiroshi Kiyono).
Reference:
Title of original paper: Cationic nanogel-based nasal therapeutic HPV vaccine prevents the development of cervical cancer
Authors: Rika Nakahashi-Ouchida1,2,3,4,5, Hiromi Mori1,4, Yoshikazu Yuki1,4,6, Tomonori Machita1,4, Yuko Katakai7, Shingo Umemoto8, Yohei Uchida1,4, Tomoyuki Yamanoue1,4, Shin-ichi Sawada1,2,9, Kazuya Ishige10, Takashi Miyazaki11, Kohtaro Fujihashi1,2,3,4,5,12,13,14, Kazunari Akiyoshi9, Yasuhiro Yasutomi15, Kei Kawana16, and Hiroshi Kiyono1,2,3,5,17,18,19
Affiliations:
- Department of Human Mucosal Vaccinology, Chiba University Hospital
- Synergy Institute for Futuristic Mucosal Vaccine Research and Development (cSIMVa), Chiba University
- Research Institute of Disaster Medicine, Chiba University
- Division of Mucosal Vaccines, International Research and Development Center for Mucosal Vaccines, The Institute of Medical Science, The University of Tokyo
- Division of Mucosal Immunology, IMSUT Distinguished Professor Unit, The Institute of Medical Science, The University of Tokyo
- HanaVax Inc.
- Department of Medical Science Project Planning and Support, The Corporation for Production and Research of Laboratory Primates
- Faculty of Medicine, Department of Otorhinolaryngology, Head and Neck Surgery, Oita University
- Department of Polymer Chemistry, Faculty of Engineering, Kyoto University
- Biochemicals Division, YAMASA Corporation
- Toko Yakuhin Kogyo Co., Ltd.
- Division of Clinical Vaccinology, International Research and Development Center for Mucosal Vaccines, The Institute of Medical Science, The University of Tokyo
- Division of Mucosal Vaccines, International Vaccine Design Center, The Institute of Medical Science, The University of Tokyo
- Department of Pediatric Dentistry, The University of Alabama at Birmingham
- Laboratory of Immunoregulation and Vaccine Research, Tsukuba Primate Research Center, National Institutes of Biomedical Innovation, Health and Nutrition
- Department of Obstetrics and Gynecology, Nihon University School of Medicine
- Institute for Advanced Academic Research, Chiba University
- Future Medicine Education and Research Organization, Chiba University
- CU-UCSD Center for Mucosal Immunology, Allergy and Vaccines (cMAV), UC San Diego School of Medicine
Journal: Science Translational Medicine
DOI: 10.1126/scitranslmed.ado8840
Contact: Rika Nakahashi-Ouchida
Chiba University Hospital, Chiba University, Japan
Email: rnakahashi@chiba-u.jp
Academic Research & Innovation Management Organization (IMO), Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522, Japan
Email: cn-info@chiba-u.jp
Recommend
-

Examining Social Injustice of Modern Society through “Child Poverty”: Creating a Society Where Every Parent is Respected, and Every Child Thrives
2023.05.26
-

Unlocking the Enigma of Dark Matter: Gazing Beyond the Lens into the Universe
2023.06.09
-

Enigmatic Soliton Waves in Nature and Space: Demystifying Equations of Everyday Physical Phenomena
2023.12.04







