Researchers discover that some antibody-based therapies can have off-target effects, impairing the immune system’s ability to fight off cancer
Immune checkpoint inhibitors have revolutionized cancer treatment, but not all patients respond equally. Now, researchers from Japan have explored why two anti-PD-L1 antibodies, which target the same immune pathway, produce vastly different therapeutic outcomes in a mouse cancer model. They found that an immune mechanism known as antibody-dependent cellular cytotoxicity can inadvertently destroy antitumor immune cells. These findings underscore the importance of selecting antibody drugs that minimize off-target effects to improve the efficacy of immunotherapy.
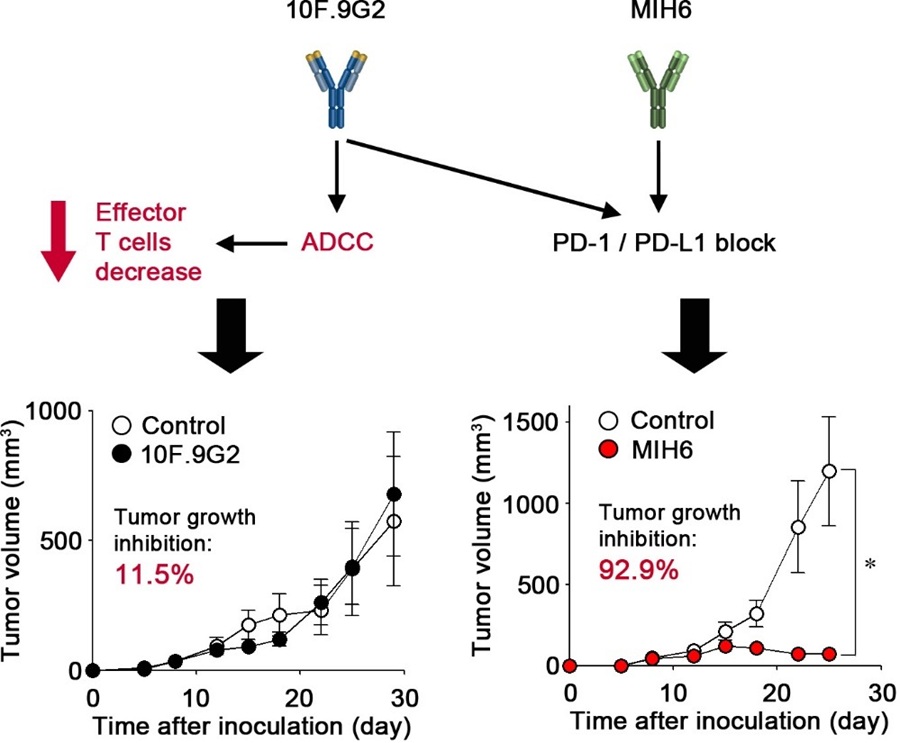
Image title: Two monoclonal antibodies with the same target exhibit markedly different antitumor effects
Image caption: 10F.9G2, a monoclonal antibody with high antibody-dependent cellular cytotoxicity, has an off-target effect that involves the reduction of CD8+ T cells. This surpasses the on-target effect, namely programmed cell death protein 1/programmed death-ligand 1 axis inhibition, resulting in no observable antitumor efficacy. In contrast, MIH6, which has low antibody-dependent cellular cytotoxicity activity, exerts only the on-target effect, leading to an effective antitumor response.
Image credit: Assistant Professor Yuta Tamemoto from Chiba University, Japan
Source link: https://www.sciencedirect.com/science/article/pii/S0378517325005927?via%3Dihub
Image license: CC BY 4.0
Usage Restrictions: Credit must be given to the creator.
Immune checkpoint inhibitors (ICIs), a powerful form of immunotherapy, have revolutionized cancer treatment by unleashing the body’s own immune system to fight tumors. These compounds target the programmed cell death-ligand 1 (PD-L1), a surface protein typically found on tumor cells, which enables the tumors to avoid recognition by immune T cells. By disrupting PD-L1’s function with specially tailored antibodies, ICI-based strategies have brought hope to countless patients with cancer. However, despite their undeniable success, these treatments do not work for everyone. Many patients remain unresponsive to immunotherapy, and scientists have been struggling to understand why some people benefit while others don’t.
While much research has focused on tumor and patient characteristics that could influence treatment response, less attention has been paid to how the drugs themselves might influence their treatment success. Different antibody drugs, even those targeting the same immune pathway, may have varying properties that subtly or dramatically impact their effectiveness. These include differences in how long they stay in the body, how well they reach tumors, and perhaps most importantly, what other cellular functions they might trigger beyond their intended target.
Against this backdrop, a research team led by Assistant Professor Yuta Tamemoto and Professor Hiroto Hatakeyama from the Graduate School of Pharmaceutical Sciences at Chiba University, Japan, investigated what factors affect the performance of anti-PD-L1 antibodies. Their findings were made available online on May 22, 2025, and were published in Volume 679 of the International Journal of Pharmaceutics on June 30, 2025.
The researchers set out to understand why two different anti-PD-L1 monoclonal antibodies, both designed to block cancer’s immune evasion via the same mechanism, showed vastly different results in laboratory models. In particular, they focused on a powerful immune response called antibody-dependent cellular cytotoxicity (ADCC). Simply put, ADCC is a mechanism that triggers when a cell is covered in antibodies; this elicits a strong immune response that leads to the death of the cell, usually mediated by natural killer cells.
The team compared two specific anti-PD-L1 monoclonal antibodies: MIH6, which has minimal ADCC activity, and 10F.9G2, which exhibits strong ADCC activity. In a mouse tumor model, MIH6 was remarkably effective, inhibiting tumor growth by over 90%. In contrast, 10F.9G2 showed only a slight effect on tumor growth, despite targeting the same immune pathway. Initially, the researchers investigated whether differences in how the antibodies bound to target cells or how they moved through the body could explain this disparity. While MIH6 bound more strongly to cancer cells and remained in the bloodstream longer, these differences alone were not enough to account for the drastic differences in treatment outcomes.
Turning to ADCC as a possible explanation, the researchers discovered that 10F.9G2, the one with strong ADCC activity, unexpectedly reduced the number of antitumor immune cells called CD8+ T cells. This happens because PD-L1, the target of these antibodies, is present not only on cancer cells but also on healthy T cells. When antibodies with high ADCC activity bind to PD-L1 on T cells, they inadvertently trigger the destruction of an essential component of the immune system.
This finding reveals that while ADCC is often a desired secondary mechanism for killing cancer cells in ICI therapies, it can cause a detrimental ‘off-target’ effect when targeting immune checkpoint molecules. “Our results highlight the critical need to consider ADCC activity when designing or selecting antibody therapeutics for immune checkpoint blockade, especially in cancer immunotherapy,” says Dr. Tamemoto.
By shedding light on this unwanted side effect, this study could help scientists improve ICI-based therapies through the careful selection of antibody features based on patient characteristics at the molecular level. “If we assess PD-L1 expression on T cells and determine whether anti-PD-L1 monoclonal antibodies with ADCC activity are appropriate in each case, it may be possible to select the optimal antibodies for each patient,” explains Dr. Tamemoto. “By engineering antibodies that avoid damaging essential immune cells, we may be able to minimize side effects and maximize the effectiveness of cancer immunotherapy.”
Further research efforts into these mechanisms may pave the way for improved cancer treatment.
About Assistant Professor Yuta Tamemoto from Chiba University
Dr. Yuta Tamemoto obtained a PhD degree in pharmacy from the Graduate School of Pharmaceutical Sciences at Chiba University in 2023. He currently serves there as Assistant Professor, conducting research on the topics of life sciences and clinical pharmacy, which includes antibody-based therapies, molecular drug interactions, pharmacokinetics, and pharmacodynamics, among others. He currently has eight scientific publications to his name, with more than 30 citations.
Funding:
This work was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant Numbers 19H03387 and 22H02778] (HH), the Takeda Science Foundation (HH), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (HH), the Uehara Memorial Foundation (HH), and the Nakatomi Foundation (HH).
Reference:
Title of original paper: Antibody-dependent cellular cytotoxicity of anti-programmed death-ligand 1 antibodies for T cells attenuate their antitumor efficacy in a murine tumor model
Authors: Yuta Tamemoto1,2, Yoshito Nakamura1,2, Taiki Kurino2, Ruiheng Tang1, Takahiro Arai2, Shogo Yasuda1, Riho Kume1, Hiroyuki Suzuki3, Tomoya Uehara3, Hidetaka Akita4, Akihiro Hisaka2, and Hiroto Hatakeyama1,2
Affiliations:
- Laboratory of DDS Design and Drug Disposition, Graduate School of Pharmaceutical Sciences, Chiba University
- Laboratory of Clinical Pharmacology and Pharmacometrics, Graduate School of Pharmaceutical Sciences, Chiba University
- Laboratory of Molecular Imaging and Radiotherapy, Graduate School of Pharmaceutical Sciences, Chiba University
- Laboratory of DDS Design and Drug Disposition, Graduate School of Pharmaceutical Sciences, Tohoku University
Journal: International Journal of Pharmaceutics
DOI: 10.1016/j.ijpharm.2025.125755
Contact: Hiroto Hatakeyama
Graduate School of Pharmaceutical Sciences, Chiba University
Email: h-hatakeyama@chiba-u.jp
Academic Research & Innovation Management Organization (IMO), Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: cn-info@chiba-u.jp









