Researchers around the world are in a spirited race to unravel the mystery of how life begins. Among the many breakthroughs in artificial embryo model research—which aims to create early embryonic structures using only stem cells—one of the most significant was achieved by Lecturer Yasuhide Ohinata of the Graduate School of Medicine. He successfully established ‘Primitive Endoderm Stem cells (PrES cells),’ the final piece needed to create an embryo model. This pioneering work has been widely acclaimed for its scientific and technological impact, earning him the 2024 Minister of Education, Culture, Sports, Science and Technology’s Prize for Science and Technology (Research Category). With careful attention to ethics, Dr. Ohinata continues to explore the fundamental mechanism of life’s origins—an endeavor as delicate as it is profound.
Three types of stem cells are needed for artificial embryo models

So, how does life begin?
In mammals, life starts from a single fertilized egg. After fertilization, the egg—such as that of the mouse, a widely used model organism—divides into two, then four, eight, sixteen cells, and so on. As the cells continue to divide, they begin to differentiate into distinct types. By day 3.5 after fertilization, a structure called the blastocyst forms, composed of several dozen cells organized into three distinct cell lineages.
All three of these cell types are essential to artificially creating an embryo model. The first breakthrough came in 1981, when Professor Sir Martin Evans and colleagues succeeded in deriving embryonic stem (ES) cells, which give rise to the embryo proper, from mouse blastocysts—a discovery later recognized with the Nobel Prize. In 1998, Professor Satoshi Tanaka of the University of Tokyo and his team established trophoblast stem (TS) cells, which develop into the placenta.
However, the third lineage remained elusive for many years. Then, in 2022, our research team finally succeeded in establishing PrES cells from mouse blastocysts. With this final piece now in place, all the fundamental components necessary to build an artificial embryo model—ES, TS, and PrES cells—have been realized in theory.
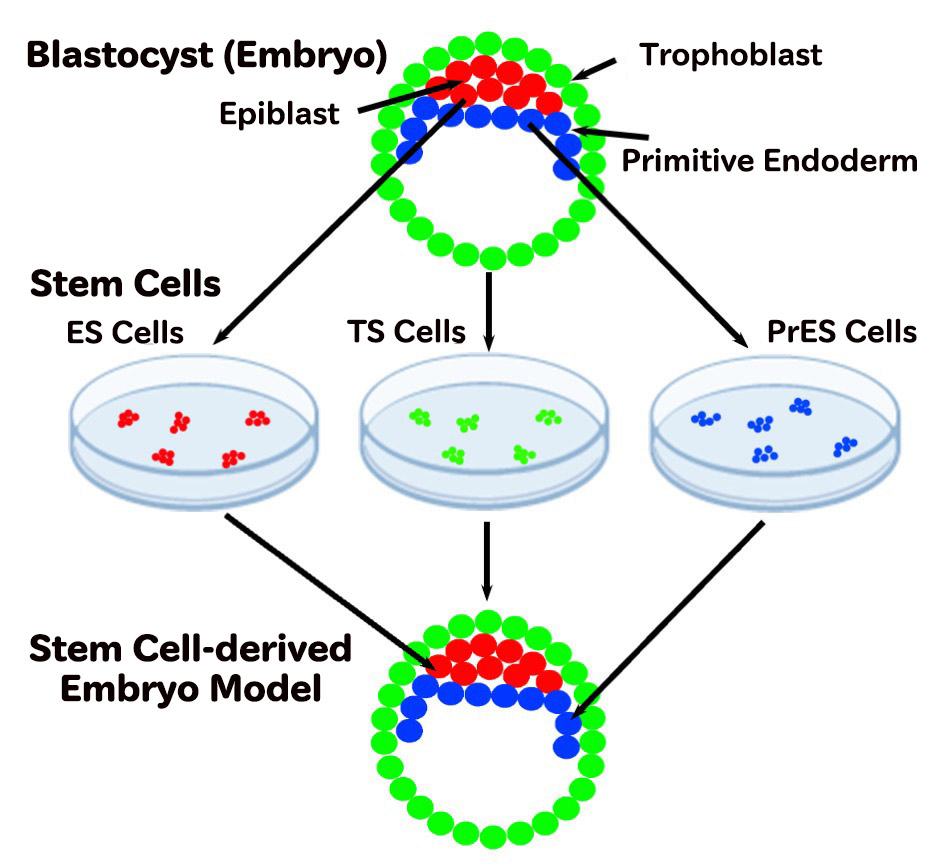
By 3.5 days after fertilization, a structure known as a blastocyst forms, consisting of several dozen cells organized into three distinct types: epiblast, trophoblast, and primitive endoderm
Our research aims to combine these three types of stem cells to elucidate the mechanisms by which the buds of life are formed. We have successfully generated embryo-like structures by assembling stem cells and implanting them into the uterus of a mouse. While these structures resemble natural embryos, they have not yet developed into viable offspring. Our ongoing efforts focused on accurately recreating this developmental process in vitro. The insights we gain from mouse models will provide a crucial foundation for understanding the earliest stages of human development.
A simple yet crucial developmental mechanism
What was the key to establishing PrES cells?
ES cells and iPS cells are known as ‘pluripotent’ cells because they can give rise to all the cell types that make up our body. These cells have garnered widespread interest among researchers. However, pluripotent cells are unable to generate extraembryonic tissues such as the placenta and yolk sac—structures that are essential for embryonic development but no longer needed after birth. We were convinced of the importance of establishing PrES cells and steadily pursued this line of research.
The process of culturing these cells was painstaking. Serum, commonly used in cell culture media, contains numerous unknown components. In some cases, serum can inhibit cell growth or alter cellular characteristics. We also had to carefully determine the optimal combination of growth factors to support cell proliferation. Through a process of trial and error—systematically addressing each challenge—we succeeded in establishing PrES cells by developing a serum-free culture medium and identifying the precise combination of factors needed for their growth.
With the rise of embryo models, is there room for success in a ‘Red Ocean’?
Embryo model research is drawing worldwide attention, and I know I can’t compete using time- and resource-intensive methods like large-scale screening. Instead, I need to carefully consider, “What is the key problem I should be solving right now?” From there, I can design a plan that’s both feasible and effective within limited resources. If I can take an approach that others have not thought of, I can compete—even with large, well-funded laboratories. To me, the essence of research lies in refining original ideas and crafting thoughtful strategies. And above all, it’s simply more enjoyable that way.
So, how does this mindset translate into practice? For example, what I want to understand first is how the control system of early development works—not every fine-grained detail of gene expression. To use a computer analogy: even if you don’t fully understand how a computer works internally, you can gradually figure out how to use it by typing on the keyboard and seeing letters appear on the screen. But if you were to disassemble the computer into individual parts, it would be almost impossible to know what each part does or how they work together.
Now, let’s take the analogy a step further and map the elements: pressing a key corresponds to a signal input, and a computer part corresponds to a gene. Trying to understand how a computer works by examining each part in isolation is like trying to understand early development by analyzing the role of each gene separately—it’s an enormous task that demands tremendous time and effort.
However, if you focus on which keys are pressed, and when—in other words, the combination and timing of signal inputs—you can drastically simplify the experimental approach. Of course, we can still obtain gene expression and epigenome data when needed. But by starting from signal-level information and observing how different combinations influence cell behavior, we can design feasible research.
Reconstructing the logic of life: From cells to embryos

The reason to study the origins of life is to uncover the underlying logic that sustains its continuity—and ultimately—to lay the foundation for transformative advances in infertility treatment
No matter how many cells you grow in a dish, it can be difficult to truly regard them as ‘life’ itself. Rather than treating cells and embryos as entirely separate entities, I want to understand the transition from cells to life as a continuous process. By grasping this logic, I aim to reconstruct that progression using stem cells in a test tube.
This line of research may have direct applications in reproductive medicine. Japan currently performs one of the highest numbers of infertility treatments in the world. Among the many causes of infertility, egg aging presents a major challenge. The current standard approach is to retrieve the few remaining viable eggs, fertilize them, and implant them into the uterus. However, this strategy does not address the root problem—egg aging itself. If we can apply stem cell culture techniques to recreate functional placenta and yolk sac tissues in humans, it may become possible to rejuvenate these extraembryonic structures without altering the fetus’s genetic information. While this is still an unexplored field, I am conducting it with the hope that it will one day contribute to breakthroughs in assisted reproductive technologies.
Since this research touches on the very origins of life, ethical considerations are unavoidable.
Currently, I conduct my work as an embryologist using mouse models. However, if I were to pursue embryo model research using human cells, I believe it would be essential to approach it with even greater ethical sensitivity than with animal studies. We must remain consistently aware of ethical concerns, even more so than when using mice, and clearly articulate the purpose of the research to ensure its social acceptability.
Take, for example, the case of organ transplantation: today’s guidelines were established only after decades of careful and repeated public discussion. I believe embryo model research must also begin with thorough deliberation. Guidelines should be seen not as obstacles, but as frameworks that protect researchers. It is only under appropriate regulations that we can carry out ethically sound research. That is why establishing such rules is extremely important—and why I hope they will be both practical for researchers and acceptable to society*.
*Currently, only a few countries have established laws and guidelines in this area. In Japan, an expert committee has just recently recommended to the government that such rules be developed.
Life doesn’t always go as you planned—and that’s not necessarily bad

After graduating from high school, he pursued a career as a jazz trumpeter before entering university—an unconventional path. His motto, “An unexpected detour. That’s will do, too!” reflects both his approach to life and his attitude towards research.
I’ve never thought of myself as particularly talented at research. When I was a graduate student, I often worried about what I needed to do for good research. Personally, I took a strict approach: I focused solely on my work and simply tried to put in more effort than others. However, how you approach research really depends on the individual, and I believe each person should choose a path with which they can feel content. In my case, I try to carefully evaluate the cards I’ve been dealt and prioritize what matters most to me. It’s hard to have everything at once.

From personal experience, I can say that life rarely goes the way you expect. I had many difficult moments in grad school—times when the efforts I thought I had made weren’t rewarded. But looking back now, those experiences weren’t so bad after all. In fact, I can even say they were enjoyable in the end. Taking a detour is not the end of the world. The path to becoming a researcher is far from smooth, but as long as you keep asking yourself, “What do I really want to understand, and how can I find it out?” and keep moving forward—even if it’s just a little at a time—you will eventually find your way. That kind of calm determination is incredibly important. So if you ever feel stuck in your research, I hope you remember these words from someone who’s just a bit further down the road.

● ● Off Topic ● ●
I heard that cooking is one of your hobbies.
At first, I just wanted to try making restaurant-quality food at home. Before I knew it, it had become a regular thing to invite friends over and treat them to good meals. I also enjoy sourcing ingredients directly from nature. The other day, I went offshore fishing in Hitachi with a professor from Jichi Medical University, and we caught a lot of mackerel. That same professor also has a hunting license. One day, I came home to find a cardboard box at my door—with several ducks inside! (laughs) I made soba noodles with roasted ducks and enjoyed every bite with gratitude.

With your busy research schedule, how do you find time for hobbies like that?
I’m basically a workaholic. But when I’m feeling stuck or not getting the results I want, I think it’s really important to take care of my mental well-being, too. When I need a quick pick-me-up, nothing beats a bowl of Ramen. Another of my mottoes is: Work hard, eat well—and when the chance comes, dive into fun with full focus.
Recommend
-

The Global Goal of Carbon Neutrality by 2050 (Part 1): Universities as Agents of Change
2023.07.12
-

Taking up Immunological Research against Incurable Diseases−The Significance of “1” Life
2023.01.12
-

Reassessing the role of “Forest Resources” in achieving carbon neutrality: Measuring forests with the combination of drones, mathematics, and computer graphics
2023.09.12