Purine molecules, including caffeine, increase HER activity up to four times, paving the way for affordable, large-scale hydrogen production
High costs have long held back hydrogen production from water, with electrolyzers priced at $2,000–$2,600 per kilowatt in 2024. Now, researchers from Japan have found that modifying platinum cathodes with naturally occurring purine bases can boost the hydrogen evolution reaction (HER) activity, the key step where water is split into hydrogen, up to four times. This approach can significantly reduce platinum requirements, bringing affordable, large-scale hydrogen production closer to reality.
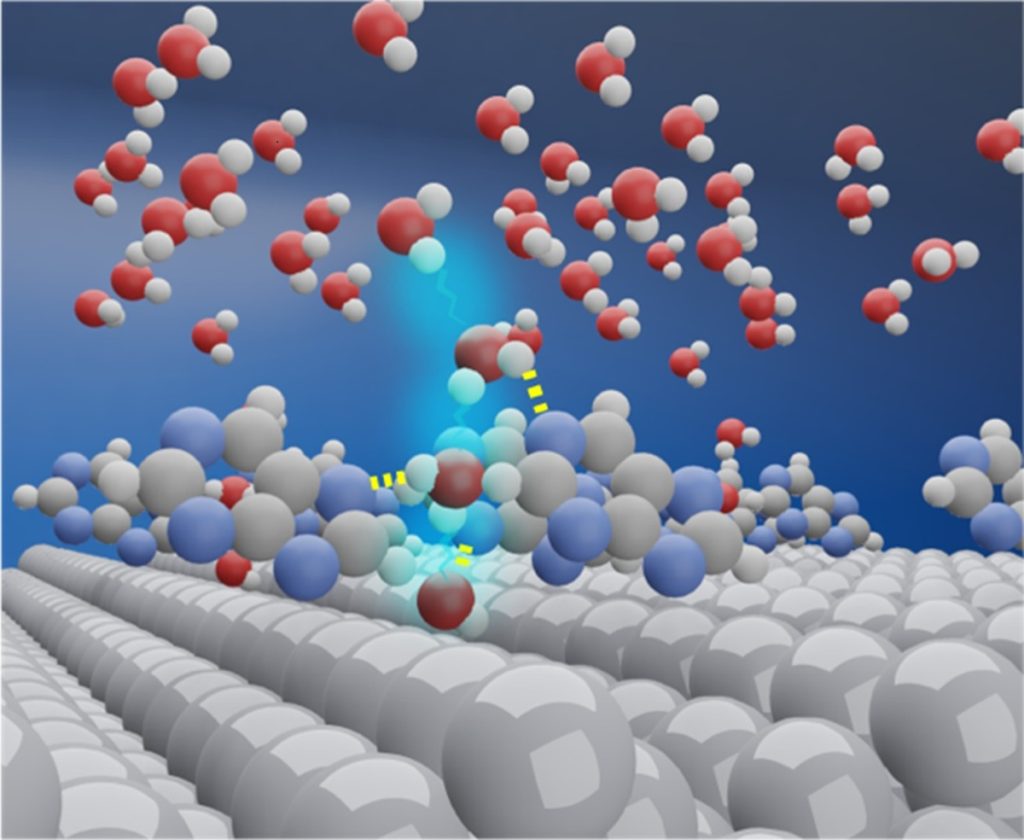
Image title: Purine-modified platinum electrodes boost hydrogen production
Image caption: Researchers from Japan enhanced the hydrogen evolution reaction (HER) by modifying platinum cathodes with purine bases. The purine molecules form hydrogen-bonded cages with surrounding water, which helps remove hydroxide ions from the surface, lowering the energy barrier and increasing HER activity up to four times.
Image credit: Dr. Syunnosuke Tanaka and Prof. Masashi Nakamura from Chiba University, Japan
Image license: Original Content
Usage Restrictions: Cannot be reused without permission.
Hydrogen production from water may seem straightforward: splitting water into hydrogen and oxygen gases using an electric current. However, one major challenge is the significant cost associated with it. To scale up this technology, the U.S. Department of Energy has set a target of $1/kg H2 by 2030, with an interim target of $2/kg H2 by 2025. Analysts note that achieving these targets will require substantial reductions in electricity costs as well as improvements in electrolyzer (device that performs electrolysis) efficiency.
In line with these requirements, researchers from Japan have discovered a way to reduce the platinum requirements needed for cathodes in water electrolysis by adding purine bases— organic molecules found in DNA and RNA. The modification increases the hydrogen evolution reaction (HER) activity by 4.2 times, significantly lowering the need for costly platinum. The work led by Dr. Syunnosuke Tanaka and Professor Masashi Nakamura from the Graduate School of Engineering, Chiba University, Japan, could pave the way for more practical and affordable water electrolysis systems. Their findings were made available online on September 04, 2025, and published in Volume 172 of the International Journal of Hydrogen Energy on September 26, 2025.
“Platinum has limited resources, which is an issue for the widespread use of water electrolysis and fuel cell catalysts. The significant reduction of platinum loading in electrochemical devices will be an important step toward the practical application of catalysts for water electrolysis,” says Prof. Nakamura.
During water splitting, hydrogen ions (H+) adsorb onto the catalyst and then combine to form hydrogen gas. In alkaline media, where H+ ions are not readily available, the catalyst must first break the OH bond of water to generate adsorbed hydrogen, an additional step that requires more energy. Caffeine, a purine base, is known to promote the breakup and adsorption of hydrogen at the cathode under alkaline conditions. Building on this, the researchers studied HER on single-crystal platinum electrodes modified with other purine bases, including caffeine, xanthine, purine, theophylline, and theobromine.
Their experiments showed that purine and theophylline enhanced HER the most, increasing activity by 4.2 and 5 times, respectively. The effect of the purine bases depended on their chemical structure: molecules with methyl groups, such as caffeine, theophylline, and theobromine, enhanced HER at moderate coverage but aggregated at higher coverage, blocking the active sites. In contrast, purine and xanthine, which lack methyl groups, did not aggregate and steadily increased the HER activity.
X-ray diffraction and density functional theory calculations revealed the reason for this enhancement. When purine is adsorbed on the platinum surface, water molecules around the purine form hydrogen bonds with the purine’s nitrogen atoms, creating a cage-like structure. This structure facilitates the transfer of OH– ions away from the surface, rapidly clearing them away and lowering the energy barrier for HER.
When applied to commercial platinum/carbon (Pt/C) catalysts, a purine-modified Pt/C achieved 3.2 times higher HER activity than its unmodified catalyst in 0.1 M alkaline lithium hydroxide solution. This development could make hydrogen production far more affordable by using a naturally occurring, widely available substance to enhance the activity of expensive electrodes. Furthermore, this strategy could bring us one step closer to achieving the 2030 energy cost targets for hydrogen production via water electrolysis.
“Water electrolysis using renewable energy is necessary to achieve carbon neutrality. The highly active electrode catalyst developed in this study will lead to cost reductions and improved energy conversion efficiency of water electrolysis systems,” says Prof. Nakamura.
To see more news from Chiba University, click here.
About Professor Masashi Nakamura from Chiba University, Japan
Dr. Masashi Nakamura is currently a Professor at the Department of Applied Chemistry and Biotechnology, Graduate School of Engineering, Chiba University, Japan. He obtained his Ph.D. from Keio University in 2002. He is a member of the Chemical Society of Japan, the Electrochemical Society of Japan, and the Japan Society of Vacuum and Surface Science. He has over 100 publications and over 3,500 citations to his name. His research interests include surface science and surface electrochemistry.
Funding:
This study was supported by the Sasakawa Scientific Research Grant, JST SPRING (JPMJSP2109), the ENEOS Hydrogen Trust Fund, the Steel Foundation for Environmental Protection Technology, and the Japan Society for the Promotion of Science (JSPS/KAKENHI, 23K23155).
Reference:
Title of original paper: Active site of the Pt electrodes modified with purine bases for hydrogen evolution reaction in alkaline solution
Authors: Syunnosuke Tanakaa, Hiroaki Hattoria, Tomoyuki Koganezawab, Tomotaka Nakatanib, Rosantha Kumarab, Osami Sakatab, Kenji Miyatakec,d, Nagahiro Hoshia, and Masashi Nakamuraa
Affiliations: aDepartment of Applied Chemistry and Biotechnology, Graduate School of Engineering, Chiba University
bCenter for Synchrotron Radiation Research, Japan Synchrotron Radiation Research Institute (JASRI)
cClean Energy Research Center, University of Yamanashi
dDepartment of Applied Chemistry, Waseda University
Journal: International Journal of Hydrogen Energy
DOI: 10.1016/j.ijhydene.2025.151318
Contact: Masashi Nakamura
Graduate School of Engineering, Chiba University, Japan
Email: mnakamura@faculty.chiba-u.jp
Academic Research & Innovation Management Organization (IMO), Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 Japan
Email: cn-info@chiba-u.jp
Recommend
-

The role of the Research Institute of Disaster Medicine (RIDM): The indispensable research hub for urban digital transformation
2023.02.23
-

Children-centered support and school operations: On the occasion of the founding of the Children and Families Agency
2023.02.15
-

Predict, Classify, Treat: AI-Driven Therapeutic Science in Action
2025.12.12







