Researchers develop a novel plant regeneration approach by modulating the expression of genes that control plant cell differentiation
Conventional plant regeneration approaches by cell culture require the external application of plant growth regulators, including hormones. However, optimizing culture conditions can be laborious. Now, researchers have developed a novel plant regeneration system that omits the need for hormone application by genetically regulating the expression of genes that control plant cell differentiation. Their work holds significant potential in the development of genetically modified plants in a simpler and cost-effective manner.
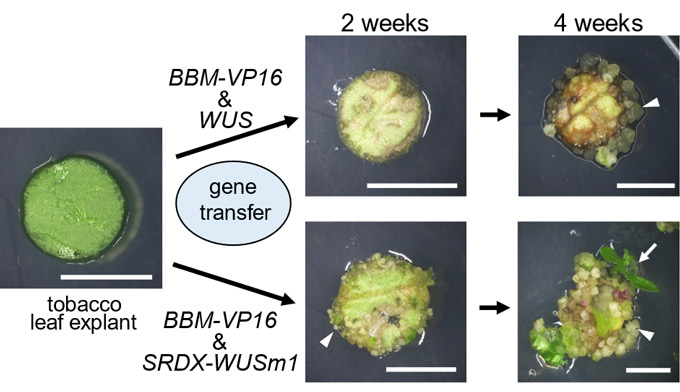
Image title: Researchers develop novel gene expression-based plant regeneration approach without the application of plant growth regulators
Image caption: Conventional plant regeneration techniques require the application of plant growth regulators, like hormones, which can be species-specific and laborious. In a new study, researchers have developed a novel system of plant regeneration by modulating the function and the expression of genes involved in plant cell dedifferentiation (cell proliferation) and redifferentiation (organogenesis).
Image credit: Associate Professor Tomoko Igawa, Chiba University, Japan
Image license: CC BY 4.0 DEED
Usage restrictions: Credit must be given to the creator.
For ages now, plants have been the primary source of nutrition for animals and mankind. Additionally, plants are used for the extraction of various medicinal and therapeutic compounds. However, their indiscriminate use, along with the rising demand for food, underscores the need for novel plant breeding practices. Advances in plant biotechnology can address the problems associated with food scarcity in the future by enabling the production of genetically modified (GM) plants with higher productivity and resilience to the changing climate.
Naturally, plants can regenerate an entire new plant from a single ‘totipotent’ cell (a cell that can give rise to multiple cell types) through dedifferentiation and redifferentiation into cells with various structures and functions. Artificial regulation of such totipotent cells through plant tissue culture is widely used for plant conservation, breeding, generation of GM species, and scientific research purposes. Conventionally, tissue culture for plant regeneration requires the application of plant growth regulators (PGRs), such as auxins and cytokinins, to control cell differentiation. However, optimum hormone conditions can vary significantly with plant species, culture conditions, and tissue type. Therefore, establishing optimum PGR conditions can be time-consuming and laborious.
To overcome this challenge, Associate Professor Tomoko Igawa, along with Associate Professor Mai F. Minamikawa from Chiba University, Professor Hitoshi Sakakibara from the Graduate School of Bioagricultural Sciences, Nagoya University, and Expert Technician Mikiko Kojima from RIKEN CSRS, have developed a versatile method of plant regeneration by modulating the expression of ‘developmental regulator’ (DR) genes which control plant cell differentiation. Giving further insights into their research work published on 3 April 2024 in Volume 15 of Frontiers in Plant Science, Dr. Igawa says, “Instead of using external PGRs, our system uses the DR genes, which are involved in development and morphogenesis, to control cellular differentiation. The system utilizes transcription factor genes and resembles induced pluripotent cell generation in mammals.”
The researchers ectopically expressed two DR genes, namely — BABY BOOM (BBM) and WUSCHEL (WUS) from Arabidopsis thaliana (used as the model plant), and examined their effects on the differentiation of tobacco, lettuce, and petunia tissue cultures. BBM encodes a transcription factor that regulates embryonic development, while WUS encodes a transcription factor that maintains stem cell identity in the shoot apical meristem region.
Their experiments revealed that the expression of Arabidopsis BBM or WUS alone was insufficient to induce cell differentiation in tobacco leaf tissue. Conversely, co-expression of functionally enhanced BBM and functionally modified WUS induced an accelerated and autonomous differentiation phenotype. The transgenic leaf cells differentiated into calli (a disorganized mass of cells), greenish organ-like structures, and adventitious shoots in the absence of PGR application. Quantitative polymerase chain reaction (qPCR) analysis (a technique used to quantify gene transcripts) revealed that the expression of Arabidopsis BBM and WUS was associated with the formation of transgenic calli and shoots.
Given the key role of phytohormones in cell division and differentiation, the researchers went on to quantify the levels of six phytohormones, namely — auxins, cytokinins, abscisic acid (ABA), gibberellins (GAs), jasmonic acid (JA), salicylic acid (SA), and their metabolites in the transgenic plant cultures. Their findings revealed that the levels of active auxins, cytokinins, ABA, and inactive GAs increased as cells differentiated to form organs, highlighting their role in plant cell differentiation and organogenesis.
Furthermore, the researchers used transcriptome by RNA sequencing (a technique used for qualitative and quantitative analysis of gene expression) to assess the gene expression patterns in the transgenic cells showing active differentiation. Their results suggested that genes related to cell proliferation and auxins were enriched among the differentially upregulated genes. Further validation using qPCR revealed that four genes were upregulated or downregulated in the transgenic cells, including those regulating plant cell differentiation, metabolism, organogenesis, and auxin response.
Overall, these findings shed light on the novel and versatile approach to plant regeneration without the need for externally applying PGR. Moreover, the system used in this study has the potential to advance our understanding of the fundamental processes of plant cell differentiation and improve the biotechnological breeding of useful plant species.
Highlighting the applications of their work, Dr. Igawa remarks, “The reported system can improve plant breeding by providing a tool to induce cellular differentiation of GM plant cells without PGR application. Therefore, in societies where GM plants are accepted as products, it would accelerate plant breeding and reduce associated production costs.”
About Associate Professor Tomoko Igawa
Dr. Tomoko Igawa is an Associate Professor at the Graduate School of Horticulture, Plant Molecular Science Center, and Research Center for Space Agriculture and Horticulture at Chiba University, Japan. Her research interests include plant sexual reproduction and development, as well as plant biotechnology. Her work focuses on understanding the molecular mechanisms underlying the sexual reproduction and differentiation of plant cells using various transgenic systems. She has authored several publications in these domains and is a member of The Japanese Society for Plant Biotechnology, The Botanical Society of Japan, Japanese Society of Breeding, The Japanese Society of Plant Physiologists, and The International Association of Sexual Plant Reproduction Research.
Funding: This work was supported by Japan Society for the Promotion of Science KAKENHI Grants (JP17K07600 to TI, JP19H04852 to TI, JP23H04732 to TI) and JST SPRING grants (JPMJSP2109 to YS).
Reference:
Title of original paper: Autonomous differentiation of transgenic cells requiring no external hormone application: the endogenous gene expression and phytohormone behaviors
Authors: Yuka Sato1, Mai F. Minamikawa2, Berbudi Bintang Pratama1, Shohei Koyama1, Mikiko Kojima3, Yumiko Takebayashi3, Hitoshi Sakakibara3,4 and Tomoko Igawa1,5,6
Affiliations:
- Plant Cell Technology Laboratory, Graduate School of Horticulture, Chiba University, Matsudo, Japan
- Institute for Advanced Academic Research (IAAR), Chiba University, Chiba, Japan,
- RIKEN Center for Sustainable Resource Science, Yokohama, Japan
- Graduate School of Bioagricultural Sciences, Nagoya University, Nagoya, Japan
- Plant Molecular Science Center, Chiba University, Chiba, Japan
- Research Center for Space Agriculture and Horticulture, Chiba University, Matsudo, Japan
Journal: Frontiers in Plant Science
DOI: 10.3389/fpls.2024.1308417
Contact: Tomoko Igawa
Associate Professor, Chiba University
Email: tigawa@chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018
Recommend
-

Unlocking Untapped Urban Resources: Driving Carbon Neutrality through Life Cycle Assessment
2023.06.29
-

“Making Bread from Plastic”: A Dream Recycling System for a Sustainable Future
2023.05.11
-

Solving Real-World Healthcare Challenges: The Power of Medical-Engineering Collaboration in Research and Education
2023.03.23



