Japanese researchers show how the glycan modification of intestinal mucins prevents obesity and inflammation in a murine model
Mucins are glycoproteins present in the gastrointestinal lining that protect the body against external invasions. Although mucin dysfunction is known to trigger disease and inflammation, the underlying mechanisms have remained elusive thus far. A recent study led by a Japanese research team describes how the N-acetylglucosamine (GlcNAc)-6-O-sulfation of O-glycans, the modification of the glycan structures found in mucins, helps combat obesity and intestinal inflammation in a murine model.
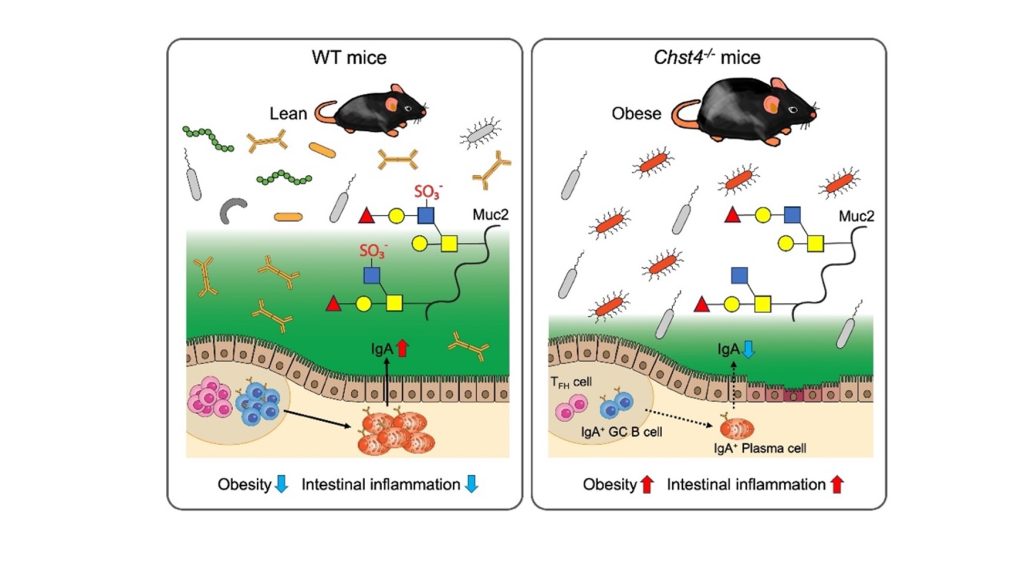
Image Title: Study highlights the protective role of intestinal sulfated glycans in obesity and intestinal inflammation
Image Caption: Chst4–/– mice lacking GlcNAc-6-O-sulfation of the mucin O-glycans showed significant weight gain, increased intestinal inflammation, and significantly reduced immunoglobulin A (IgA) production caused by an impaired T follicular helper cell-mediated IgA response. These phenotypes are dependent on the alteration of gut microbiota in the Chst4–/– mice.
Image Credit: Authors from Chiba University, Kyoto Prefectural University of Medicine, and Georgia State University
Image Source Link to be added in the Image Credit Section of EA form: https://insight.jci.org/articles/view/165944
License type: CC BY 4.0
Located in the mucus layer that lines the gastrointestinal tract, mucins—proteins with attached sugar molecules—play a key role in combating bacterial infection and providing a safe haven to friendly gut bacteria through unknown mechanisms. Although mucin dysregulation leads to metabolic disease and intestinal inflammation, the associated mechanism remains largely unknown. To address this knowledge gap, a team of researchers in Japan explored if the N-acetylglucosamine (GlcNAc)-6-O-sulfation of O-glycans, or the chemical modification of the sugar structures found in mucins, can help combat obesity and intestinal inflammation using a mouse model. This paper was made available online on August 22, 2023, and was published in Volume 8, Issue 16 of Journal of Clinical Investigation (JCI) Insight.
Initial experiments conducted by the team revealed a significant difference between the intestinal microbiota of regular or “wild type” (WT) mice and Chst4–/–mice that lacked the gene Chst4 for the GlcNAc-6-O-sulfation of mucin glycans. The latter developed obesity and showed vulnerability to both experimental colitis—artificially induced inflammation of the colon—and colitis-associated cancer.
Subsequent experiments revealed several key findings, a major among which was the following: Chst4–/–mice showed reduced fecal levels of immunoglobulin A (IgA), an antibody produced by the immune system that is essential for protecting mucosal surfaces, including the gut, against foreign invasions. Reduced IgA levels in the murine feces, thus, indicate a compromised immune system.
When asked about their results, Professor Hiroto Kawashima from Chiba University’s Graduate School of Pharmaceutical Science, the team’s lead researcher says, “Chst4–/– mice lacking GlcNAc-6-O-sulfation of the mucin O-glycans showed significant weight gain and increased susceptibility to dextran sodium sulfate-induced colitis as well as colitis-associated cancer accompanied by significantly reduced IgA production caused by an impaired T follicular helper cell-mediated IgA response.”
Next, the team found out whether “cohousing” and “microbiota transplantation”—experiments that facilitate the transfer of bacteria between WT and Chst4–/– mice—provided any relief from the observed weight gain and increased susceptibility to colitis. As expected, restoring the healthy gut microbiota in the Chst4–/– rescued the mice from disease and boosted their immune system.
The study authors note that the findings have promising biomedical implications. For instance, enhancing sulfation may provide not only relief against intestinal inflammation but also improve the “barrier function” of epithelial tissues. Epithelial tissues are present in the lining of the skin, gastrointestinal tract, and respiratory tract, among others. They defend against harmful entities by shielding internal body compartments from the external environment.
Although further studies seem necessary, the results may indeed apply not only to obesity and colitis but also to other types of immune-mediated inflammatory diseases, such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.
The gut microbiota comprises microorganisms—bacteria, viruses, fungi, protists, archaeans, and other microbes—and related metabolites. Multiple studies acknowledge their close relationship with the immune system. Recently, intestinal microbiota have sparked enormous scientific interest due to their critical roles in protecting the body against many diseases and disorders. The current study confirms earlier findings on friendly gut bacteria while shedding light on a hitherto unknown mechanism involving their regulation by intestinal mucins.
“Collectively, our findings provide insight into the significance of host glycosylation, more specifically GlcNAc-6-O-sulfation on intestinal mucins, in protecting against obesity and intestinal inflammation via regulation of the gut microbiota,” concludes first author Hirohito Abo from the Graduate School of Pharmaceutical Sciences, Chiba University.
Kudos to the collaborating research team for unleashing the hidden mechanism of mucin dysregulation!
About Professor Hiroto Kawashima
Dr. Hiroto Kawashima serves as a Professor at Chiba University’s Graduate School of Pharmaceutical Sciences. He has more than 100 publications to his credit, including those appearing in Nature Immunology and Journal of Experimental Medicine. Prof. Kawashima has been extensively cited for his exemplary work on protein glycosylation involved in various pathophysiologies.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (grants 20K15986, 18K06505, 18K15128, and 20H03379), AMED grant 20ae0101034h0005, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Japan Foundation
for Applied Enzymology, Takeda Science Foundation, the Mizutani Foundation for Glycoscience, and the Kobayashi Foundation.
Reference:
Title: N-acetylglucosamine-6-O-sulfation on intestinal mucins prevents obesity and intestinal inflammation by regulating gut microbiota
Authors: Hirohito Abo,1 Aoi Muraki,1 Akihito Harusato,2 Tetsuya Imura,3 Maki Suzuki,1 Kohta Takahashi,1 Timothy L. Denning,4 and Hiroto Kawashima1
Affiliations:
- Laboratory of Microbiology and Immunology, Graduate School of Pharmaceutical Science, Chiba University, Chiba, Japan.
- Molecular Gastroenterology and Hepatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
- Department of Surgical Pathology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
- Institute for Biomedical Sciences, Georgia State University, Atlanta, Georgia, USA.
Journal: Journal of Clinical Investigation Insight
DOI: https://doi.org/10.1172/jci.insight.165944
Contact: Hiroto Kawashima
Graduate School of Pharmaceutical Sciences, Chiba University
Email: h-kawashima@chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018





