Researchers use knockout mice and gene expression data to show that glutaminase 2 is involved in pancreatic glucose homeostasis under hyperglycemia
The enzymatic conversion of glutamine to glutamate is an important mitochondrial energy pathway that is catalyzed by glutaminase 2 (GLS2). GLS2, abundant in the liver, is also found in pancreatic β-cells. However, its role in pancreatic islets involved in glucose metabolism remains mysterious. Now, researchers from Chiba University, Japan, have used in vivo experiments and human islet cell gene expression data to show that pancreatic β-cell GLS2 regulates glucose metabolism under the condition of hyperglycemia.
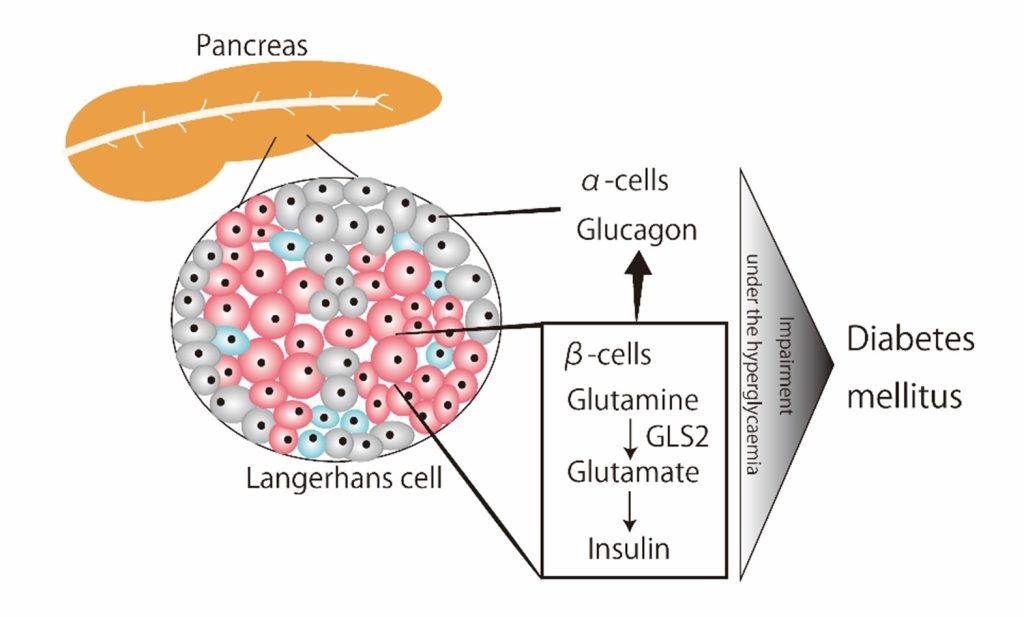
Image title: Mechanism for the onset of diabetes mellitus following GLS2 inhibition.
Image caption: Researchers from Chiba University in Japan have found that GLS2 has an important role in glucose homeostasis under conditions of hyperglycemia.
Image credit: Sawako Suzuki from Chiba University, Japan
Image license: Original content
Glutaminase 2 (GLS2) is a master regulator of glutaminolysis. GLS2 converts glutamine to glutamate, thereby playing a role in cellular energy production. GLS2 is abundant in the liver, and is also found in pancreatic β-cells. However, the role of GLS2 in pancreatic islets – in which both ɑ- and β- cells are present – associated with glucose metabolism is currently unknown.
Glucose homeostasis is known to be maintained by an intricate and complex interaction between the liver, pancreatic ɑ- cells (which make glucagon), pancreatic β-cells (that make insulin), and associated organs, such as the intestines, skeletal muscles, and fat tissue.
Researchers from Chiba University in Japan had previously identified GLS2 as a target gene for p53, thereby functioning as a tumor suppressor. Since diabetes and cancer are closely related, they decided to further examine the role of β-cells-specific GLS2 in glucose homeostasis.
In the present study, they have uncovered the role of GLS2 in glucose homeostasis. They did so by using a mouse model, in which GLS2 was conditionally deleted in pancreatic β-cells, known as Gls2 CKO mice, along with a human pancreas islet gene data repository. Their findings were published online in Scientific Reports Volume 13 on May 05, 2023. The study was led by Ms. Hannah Deguchi-Horiuchi, a doctoral student, and senior lecturer Sawako Suzuki and co-authored by Prof. Koutaro Yokote from Chiba University.
“Our previous work identified GLS2 as a target of p53, which governs ferroptosis, and showed that GLS2 had tumor suppressor function in vivo. We also knew that the GLS2 metabolite of glutamate regulated glucose-stimulated insulin production and hypothesized that the enzyme had a role to play in glucose homeostasis in pancreatic islets. This study offers important insights as diabetes and cancer may share a common molecular basis in their pathogenesis,” explains Dr. Suzuki regarding the group’s motivation to pursue the research.
The research team comparedglucose homeostasis between Gls2 knockout (Gls2 CKO) and control (RIP-Cre) mice. “When fed a high-fat diet, we found that GLS2 and p53 expression increased in pancreatic β-cells from RIP-Cre mice. Conversely, the Gls2 CKO mice developed diabetes mellitus, insulin resistance, and sustained glucose production. Paradoxically, insulin secretion was suppressed and glucagon secretion elevated despite the high blood glucose levels in Gls2 CKO mice,” says Dr. Suzuki. “This shows that impaired GLS2 activity drives the onset of diabetes mellitus by exacerbating the disrupted insulin and glucagon regulation.”
The research group’s findings were validated in separate experiments in which GLS2 was silenced in pancreatic β-cells generated from a mouse cell line. Once again, insulin secretion was seen to be reduced. Further, human pancreatic islet cell gene expression data from diabetic donors showed an increase in GLS2 expression. Lastly, in line with the Gls2 knockout mouse data, downregulated GLS2 in pancreatic β-cells from diabetic donors was paradoxically linked to suppressed insulin and enhanced glucagon gene expression.
These findings show that pancreatic β-cell GLS2 is intricately involved in regulating glucose levels under conditions of hyperglycemia. But are there any long-term therapeutic applications for this research?
Indeed, this study emphasizes that developing tailor-made diabetic medications must incorporate a patient’s genetic information along with data on blood glucose levels, metabolic status, age, duration of diabetes, and family history of the disease. Dr. Suzuki concludes, “We need to delve deeper into the GLS2-regulated glucose metabolism. Patients harboring GLS2 loss-of-function gene alterations could inherently have the potential to develop diabetes mellitus. In the future, GLS2 could even be a therapeutic target for patients suffering from this debilitating disease.”
About Associate Professor Sawako Suzuki
Dr. Sawako Suzuki is an Associate Professor in the Department of Diabetes, Metabolism, and Endocrinology at Chiba University Hospital in Japan. Dr. Suzuki has published over 50 peer-reviewed articles.
Reference:
Title of original paper: Pancreatic β-cell glutaminase 2 maintains glucose homeostasis under the condition of hyperglycaemia
Authors: Hannah Deguchi-Horiuchi1,2, Sawako Suzuki1,2, Eun Young Lee3, Takashi Miki3, Noriko Yamanaka4, Ichiro Manabe5, Tomoaki Tanaka5, Koutaro Yokote1,2
Affiliations:
- Department of Endocrinology, Hematology and Gerontology, Graduate School of Medicine, Chiba University, Japan.
- Department of Diabetes, Metabolism and Endocrinology, Chiba University Hospital, Japan.
- Department of Medical Physiology, Graduate School of Medicine, Chiba University, Japan.
- Department of Disease Biology and Molecular Medicine, Graduate School of Medicine, Chiba University, Japan.
- Department of Molecular Diagnosis, Graduate School of Medicine, Chiba University, Japan.
DOI: 10.1038/s41598-023-34336-z
Contact:
Sawako SUZUKI
Graduate School of Medicine, Chiba University
Address: 1-8-1 Inohana, Chuo, Chiba 260-8670, Japan
Email: sawakosuzuki@chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoicho, Inage, Chiba 263-8522, Japan
Email: koho-press@chiba-u.jp
Recommend
-

CUTICULA: A Secret of Unique Body Shape of Insects −Exploring the Sophisticated Mechanism Behind their Elongated and Round Bodies
2023.12.11
-

Navigating the Society-driven Research: PPI and the Quest for Research Integrity
2025.02.27
-

What causes nations and regions to perceive the COVID-19 crisis differently?〜Insights from the Humanities and Social Sciences Needed for Disaster Management Research
2022.12.12


