Scientists from Japan identify CD69, a glycoprotein present on specific immune cells, as a new target for cancer immunotherapy
Though cancer therapy is evolving, the heterogeneous nature of cancer requires innovative therapeutic targetsthat promote the anti-tumor effects of immune cells called CD8+ T cells. In this study, scientists defined an important regulator, CD69, which controls the differentiation of CD8+ T cells within tumor-draining lymph nodes, thereby regulating anti-tumor immunity. Future cancer treatment based on antibodies against CD69 could enhance anti-tumor immune responses of CD8+ T cells and lead to more efficient cancer therapies.
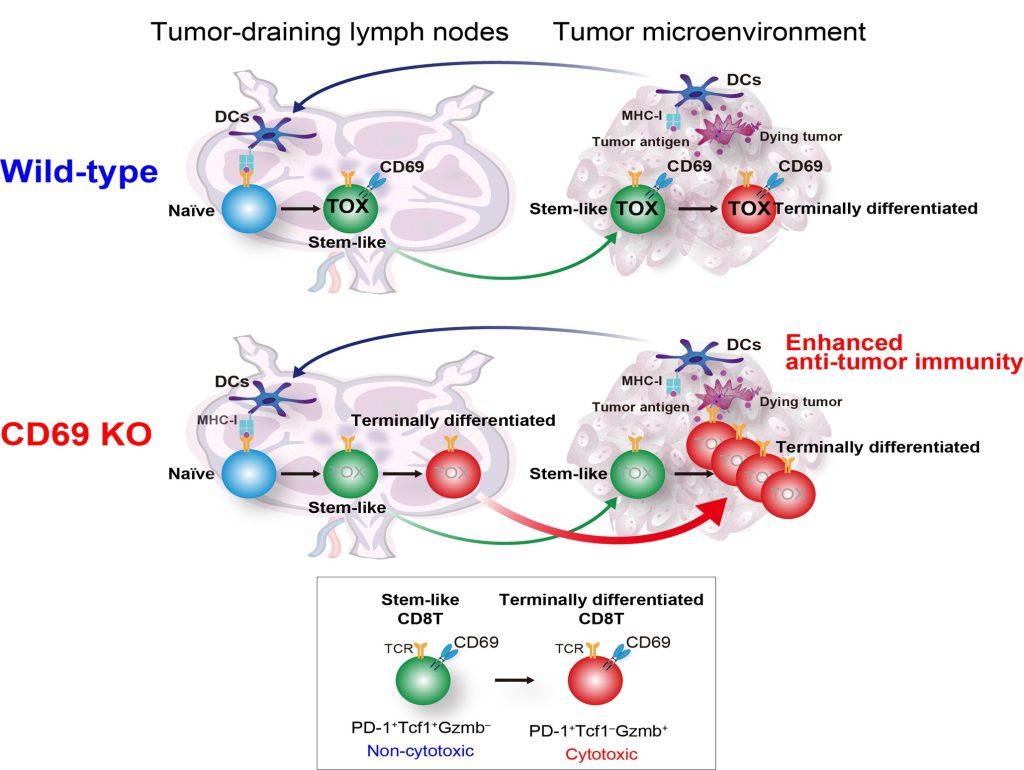
Image title: Schematic diagram to show the mechanism of CD69 action on the stem-like and terminally differentiated CD8+ T cells in wild-type and CD69-deficient mice
Image caption: A study to describe the functional mechanism of CD69 in regulating the tumor-specific CD8+ T cell fate in tumor-draining lymph nodes (TDLNs), which can be used to develop a novel anti-CD69 antibody for more efficient cancer immunotherapies in the future
Image credit: Ryo Koyama-Nasu and Motoko Y. Kimura from Chiba University
License type: Original content
CD8+ T cells, a vital component of the immune system that provides immunity against cancer, have been the focal point of anti-cancer therapies. Recent studies have identified two major subpopulations of these cells present within the tumor—the stem-like cells that do not have anti-tumor activity, and the terminally differentiated CD8+ T cells, which are generated from the stem-like cells and have cytotoxic function on tumor cells. Tumor-draining lymph nodes (TDLNs) have been found to be the primary site for the presence of these cells. However, the molecular mechanisms responsible for the generation of stem-like cells into terminally differentiated CD8+ T cells are yet to be understood.
Further, scientific studies on mice have given a glimpse into the involvement of CD69, a transmembrane functional glycoprotein often expressed on CD8+ T cells in the tumor microenvironment and the TDLNs, in anti-tumor immunity. However, the complete molecular mechanism of its involvement is not fully understood. Thus, to explore the molecular role of CD69 in anti-tumor immune responses, a team of scientists from the Graduate School of Medicine at Chiba University, Japan, carried out a study that was published on May 22, 2023, in Cancer Immunology Research, a journal of the American Association for Cancer Research. The first author of this study is Senior Lecturer Ryo Koyama-Nasu, and the team included Dr. Motoko Y. Kimura and Dr. Toshinori Nakayama of Chiba University.
To understand the relevance of CD69-targeting in boosting anti-tumor responses, the scientists used different strains of mice as tumor models in their study, including mice that do not express the CD69 molecule, i.e., CD69-deficient mice. They also evaluated the response of mice after the administration of an anti-CD69 antibody, an immune molecule that selectively binds to CD69 to inhibit its function.
Using single-cell transcriptomics, the scientists found that CD69 is a critical component that regulates the differentiation of CD8+ T cells present within TDLNs. They further observed that the deficiency of CD69 results in a reduced expression of a transcription factor, TOX—a molecule that reduces anti-tumor activity at the level of RNA regulation. Dr. Koyama-Nasu explains, “CD69 deficiency resulted in reduced expression of the transcription factor TOX in tumor-specific CD8+ T cells in TDLNs, promoting differentiation of stem-like CD8+ T cells into the functional terminally differentiated CD8+ T cells in TDLNs.”
This is a significant finding, because it explains how CD69 regulates the generation of tumor-specific CD8+ T cells in TDLNs. The team further noted that CD69-deficient mice showed increased production of terminally differentiated CD8+ T cells in the tumor microenvironment, which led to enhanced anti-tumor activity.
In this regard, Dr. Kimura, the corresponding author of the study, adds, “We observed that the use of the immune checkpoint inhibitor anti-PD-1 improves the efficiency of anti-CD69 treatment by increasing the number of available stem-like CD8+ T cells, thus making this combination-therapy effective even in the case of immunorefractory melanoma.” Moreover, CD69 deficiency or anti-CD69 treatment did not have any negative impact on the health of mice, indicating that the absence of CD69 would not negatively affect cellular functioning.
The scientists conclude that the frequent expression of CD69 on the surface of CD8+ T cells in TDLNs in human cancers makes it a novel therapeutic target for immunotherapies. Potential clinical evaluations of humanized monoclonal antibodies that recognize human CD69 could provide a new and promising strategy for future cancer immunotherapies.
About Dr. Ryo Koyama-Nasu
Dr. Ryo Koyama-Nasu has been working as a Senior Lecturer since 2021 in the Department of Experimental Immunology at the Graduate School of Medicine at Chiba University, Japan. He has 30 publications on various oncology topics in reputable peer-reviewed journals.
Reference:
Authors: Ryo Koyama-Nasu 1,2, Motoko Y. Kimura 2, Masahiro Kiuchi 1, Ami Aoki 1, Yangsong Wang 2, Yukiyoshi Mita 1, Ichita Hasegawa 2, Yukihiro Endo 2, Atsushi Onodera 1, Kiyoshi Hirahara 1, Shinichiro Motohashi 3 and Toshinori Nakayama 1
Title of original paper: CD69 imposes tumor-specific CD8+ T-cell fate in tumor-draining lymph nodes
Journal: Cancer Immunology Research
DOI: 10.1158/2326-6066.CIR-22-0406
-
Affiliations:
- Department of Immunology, Graduate School of Medicine, Chiba University
- Department of Experimental Immunology, Graduate School of Medicine, Chiba University
- Department of Medical Immunology, Graduate School of Medicine, Chiba University
Contact:
Motoko Y. Kimura
Graduate School of Medicine, Chiba University, Chiba, Japan
Address: 1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan
Email: kimuramo@chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoicho, Inage, Chiba 263-8522, Japan
Email: koho-press@chiba-u.jp
Recommend
-

Live on the Moon, Settle on Mars: Research Center for Space Agriculture and Horticulture for Space Food Production and Supply
2023.10.20
-

Creating a Flourishing Society with Novel Cultivars of Fruits: Integrating Molecular Genetics, Statistical Genetics, and Data Science for Efficient Fruit Tree Breeding
2023.09.08
-

Unfettered thinking, elaborate theorizing, and an accepting and positive attitude: With the precepts of his former supervisors in mind, Dr. Shimada is tackling the global food crisis
2023.01.04



