Researchers reveal that a high-fat diet impairs memory formation in Drosophila by disrupting the autophagy pathway.
Increased consumption of high-fat foods has been linked to cognitive decline. The underlying mechanisms, however, remain unclear. A new study by researchers from Chiba University, Japan, finds that a high-fat diet (HFD) impairs intermediate-term memory formation in Drosophila by decreasing autophagic activity. Further, HFD-induced memory deficits can be reversed by enhancing autophagic activity, opening avenues for the development of preventive treatments and autophagy-promoting lifestyle interventions to preserve cognitive function.
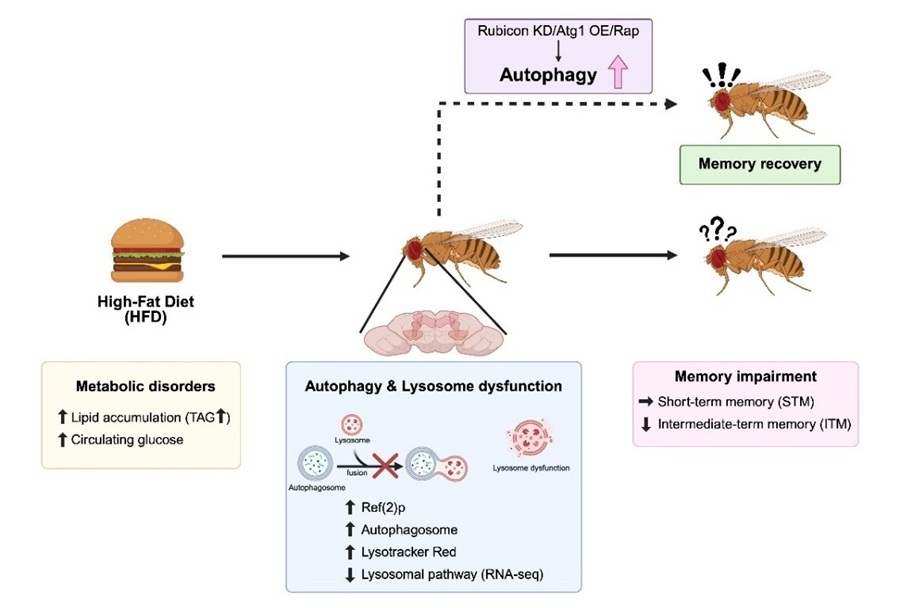
Image title: High-fat diet induces memory deficits by impairing autophagic activity
Image caption: Researchers from Chiba University have shown that high-fat diet-induced metabolic stress impairs intermediate-term memory formation in Drosophila by disrupting autophagic activity and lysosomal function.
Image credit: Associate Professor Ayako Tonoki, Chiba University, Japan
Image license: Original content
Usage restrictions: Cannot be reused without permission.
Modern lifestyles and dietary changes have significantly increased the consumption of high-fat foods, contributing to a steep rise in the prevalence of obesity, diabetes, and metabolic disorders. Furthermore, a high-fat diet (HFD) is linked to cognitive impairments and neurodegeneration and has been shown to worsen the pathology of Alzheimer’s disease—a progressive neurodegenerative condition—in mouse models. Yet, the underlying mechanisms remain largely elusive.
Autophagy, a crucial cellular recycling process, helps maintain neuronal health. Recent studies have shown that impaired autophagy contributes to neurodegeneration and cognitive decline. But is autophagy linked to HFD-induced cognitive deficits?
To address this question, researchers from Chiba University, Japan, examined the effects of the HFD on autophagy and memory formation using Drosophila, the humble fruit fly, as a model system. Rodent studies have focused mainly on specific regions of the brain, leaving the broader impact of HFDs on the nervous system unexplored. To bridge this gap, the researchers used Drosophila, given its ease of genetic manipulation, short lifespan, conserved metabolic and neural pathways with mammals, and well-validated memory assessments.
Associate Professor Ayako Tonoki and her team, including doctoral students Tong Yue, Minrui Jiang, and Kotomi Onuki from the Graduate School of Medical and Pharmaceutical Sciences, along with Professor Motoyuki Itoh from the Graduate School of Pharmaceutical Sciences, Chiba University, Japan, recently published their findings in Volume 21, Issue 8 of the journal PLOS Genetics on August 18, 2025. “Our findings suggest that diet-induced cognitive decline is not irreversible and may be improved by lifestyle interventions that promote autophagy, such as exercise or intermittent fasting. This research may raise public awareness about the cognitive risks of HFD and provide new insights into potential preventive strategies against metabolic and neurodegenerative disorders,” explains Dr. Tonoki.
The researchers maintained the flies on a normal diet or an HFD for seven days and examined their lipid—triacylglycerol (TAG) and circulating glucose levels. Notably, HFD-fed flies had significantly higher levels of TAG and glucose as well as higher intestinal lipid accumulation, suggesting that HFD altered glucose and lipid metabolism.
Next, the researchers examined the effects of HFD on memory formation by conditioning the flies to various odors and assessing their short-term (3 minutes following exposure), intermediate-term (3 hours following exposure), and long-term (24 hours following exposure) memory (STM, ITM, and LTM, respectively). Specific odor tubes were paired with an electric shock apparatus for behavioral reinforcement. Notably, HFD-fed flies exhibited impaired ITM and LTM, while STM remained unaffected.
To elucidate the role of autophagy in HFD-induced memory impairment, the researchers quantified the levels of autophagy-related proteins. They found that the levels of Ref(2)p—a protein normally degraded by autophagy—were significantly increased in HFD-fed flies. At the same time, the Atg8a-II/I ratio, a marker of autophagosome (recycling vesicles) formation, was markedly lower, indicating autophagic dysfunction. Further, temporary suppression of the autophagy protein Atg1 in adult neurons selectively reduced ITM without affecting STM, consistent with the effects observed under HFD feeding, suggesting that a temporary reduction in neuronal autophagic activity during adulthood is sufficient to cause memory decline. Conversely, boosting autophagy by overexpressing Atg1, suppressing the autophagy inhibitor Rubicon, or treating with the autophagy inducer rapamycin ameliorated memory deficits in HFD-fed flies. These findings suggest that HFD-induced memory deficits can be reversed by enhancing autophagic activity.
To better understand how HFD disrupts autophagy, the researchers examined the final stage of autophagy—the fusion of autophagosomes and lysosomes into autolysosomes, where cellular contents are degraded and recycled. Notably, HFD-fed flies showed abundant autophagosomes and lysosomes, but no change in autolysosome numbers, indicating that the HFD-induced impairment in autophagy was likely due to the defective fusion of autophagosomes and lysosomes. Interestingly, gene expression analysis revealed that lysosome signaling-related genes were significantly downregulated in HFD-fed flies. Finally, inhibition of lysosomal function markedly reduced ITM.
Overall, these findings provide novel insights into how HFD induces memory deficits through autophagic and lysosomal impairment. Addressing the cognitive risks associated with HFD can aid in the early prevention of neurodegenerative diseases.
Dr. Tonoki concludes by saying, “This research advances our understanding of how dietary habits influence brain health. Our findings may also accelerate the identification of autophagy-enhancing interventions—including specific nutrients and therapeutic agents—to combat diet-induced cognitive decline and preserve cognition in the aging population.”
To see more news from Chiba University, click here.
About Associate Professor Ayako Tonoki from Chiba University, Japan
Dr. Ayako Tonoki is an Associate Professor at the Graduate School of Pharmaceutical Sciences, Chiba University, Japan. Her research interests include neuroscience, dementia, memory, sleep, and inter-organ communication. Her work focuses on using small model organisms, such as fruit flies and zebrafish, which provide easy access to aged individuals and help elucidate the mechanisms underlying age-related decline in brain function. Dr. Tonoki is an eminent member of various academic societies.
Funding:
This work was supported by JSPS KAKENHI Grant Numbers JP22H02715, JP22H05485, and JP24K22013 for Ayako Tonoki, and JP21H02621 for Motoyuki Itoh; AMED under Grant Number JP22gm6110024h0004, and JP22gm6710006h0001 for Ayako Tonoki; and JST SPRING Grant Number JPMJSP2109 for Tong Yue and Minrui Jiang.
Reference:
Title of original paper: High-fat diet impairs intermediate-term memory by autophagic-lysosomal dysfunction in Drosophila
Authors: Tong Yue, Minrui Jiang, Kotomi Onuki, Motoyuki Itoh, and Ayako Tonoki
Affiliations: Department of Biochemistry, Graduate School of Pharmaceutical Sciences, Chiba University, Chiba, Japan
Journal: PLOS Genetics
DOI: 10.1371/journal.pgen.1011818
Contact: Ayako Tonoki
Graduate School of Pharmaceutical Sciences, Chiba University, Japan
Email: tonoki@chiba-u.jp
Academic Research & Innovation Management Organization (IMO), Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: cn-info@chiba-u.jp









